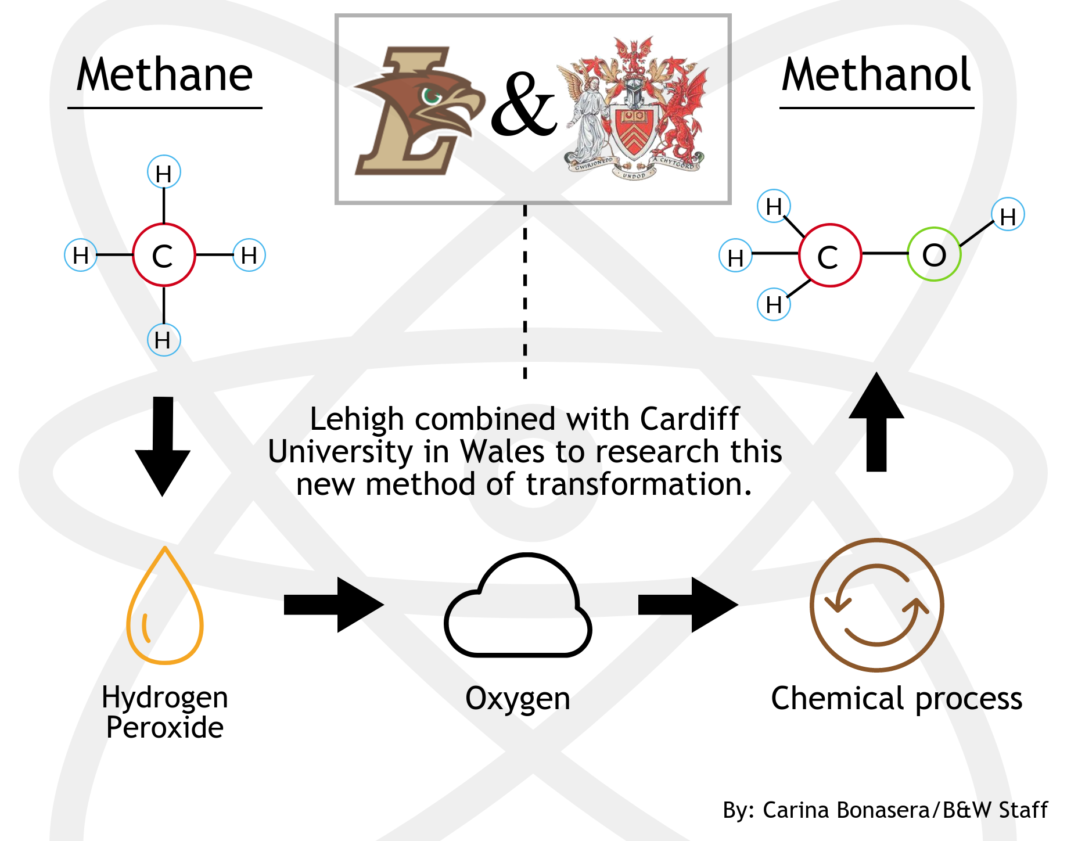
 Research fellows at Lehigh University and Cardiff University discovered a new method of converting methane to methanol.
Research fellows at Lehigh University and Cardiff University discovered a new method of converting methane to methanol.
The method, discovered through a Lehigh-Cardiff joint effort, allows for milder conditions compared to other conversion processes, resulting in an easier process at a reduced cost.
“You have to basically do a process called steam reforming,” said Qian He, a university research fellow at Cardiff University, who received his doctorate from Lehigh in 2013. “You convert methane into carbon monoxide and hydrogen. You use that, which is called steam gas, to make methanol. The process of steam reforming is very, very expensive. It requires higher temperature and high pressure, so it’s kind of an expensive process.”
The new process uses hydrogen peroxide to begin the reaction before adding oxygen, which carries out the actual process.
He said on a large scale, using hydrogen peroxide is not practical and comes with a high cost.
“Our colleague from the U.K., from Cardiff University, came up with a new idea to do that, to incorporate the variable amount of hydrogen peroxide with molecular oxygen, so we can make this reaction proceed at very mild conditions,” said Sultan Althahban, a Lehigh doctoral candidate and material science and engineering major who worked on the project with He.
The project also used gold-palladium nanoparticles as the reaction’s catalyst, or a substance that speeds up the rate of a chemical reaction.
“Usually when we use such a catalyst, that gold-palladium needs to be dispersed in like an oxide support, like titanium,” Sultan said. “But exactly in this reaction, we found that the colloid needed to be a free-floating colloid, just the gold-palladium, without using any oxide support.”
Methane, a natural gas found in vast quantities in the United States, is difficult to use as fuel because of its natural gaseous state.
He said the reason people don’t use methane as a fuel is because they don’t have a cheap way of making it.
The study that led to the new discovery was based on initial findings by Nishtha Agarwal, a doctoral student at Cardiff University. Qian He said Agarwal deserves a lot of credit because she worked closely with the catalysts and overall reaction.
“Using the catalyst method to convert is like a holy grail to the catalyst research community because methane is a very difficult molecule to work with,” he said.
Dork Sahagian, an earth and environmental science professor at Lehigh, said burning methane is better than burning oil because there is more energy per carbon dioxide molecule emitted. Carbon dioxide is released into the atmosphere through the burning of fossil fuels, and it pollutes the air and the ocean.
“In terms of pollution, methane is also much cleaner,” Sahagian said. “It’s just pure carbon and hydrogen, which burns completely to water and carbon dioxide.”
Although methane is cleaner, Sahagian said anything derived from the chemical compound is still a fossil fuel, which contributes to pollution when burned.
“(When burned), methane puts out half as much carbon dioxide per energy as coal does, but it’s still a fossil fuel,” Sahagian said. “People talk about (methane and methanol) as a transition from fossil fuel to sustainable energy sources, but there’s no question it’s still a fossil fuel.”





Comment policy
Comments posted to The Brown and White website are reviewed by a moderator before being approved. Incendiary speech or harassing language, including comments targeted at individuals, may be deemed unacceptable and not published. Spam and other soliciting will also be declined.
The Brown and White also reserves the right to not publish entirely anonymous comments.